Experience the greatest degree of in-housing milling freedom with Preat’s pre-milled titanium blanks. Our FDA-cleared PNP process was created with flexibility at each step so you can easily integrate it into your workflow. Seize the power to mill and the freedom to customize, expanding your business into the fastest growing segment of the industry!
Change The Angle. Elevate The Smile.
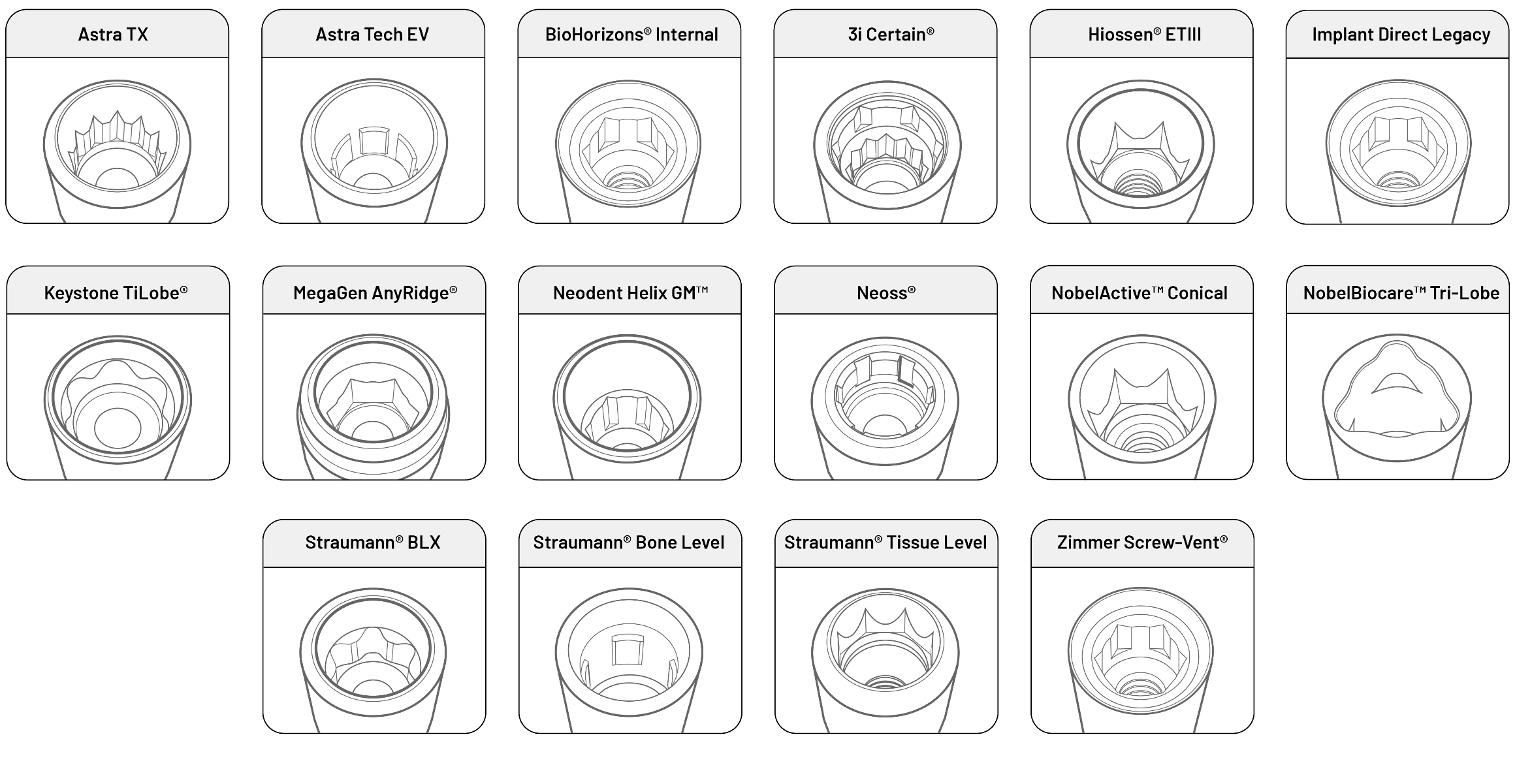
Preat’s range of pre-milled ASC blanks provides the most comprehensive implant compatibility while allowing for a variable angulated screw channel of 0-30°.

1. Some limitations for NobelBiocare® Tri-Lobe and Straumann® Bone Level-compatible connections.

Hello
Freedom to Customize. Power to Mill.
FDA Compliance Without the Cost
Enjoy the same FDA compliance as a validated milling center without the cost, time and complexity to become one. With Preat’s FDA-cleared PNP process, you simply utilize the software and equipment that has been validated for you.
Stay In-House. Stay In Control.
The PNP process lowers regulatory barriers so your lab can start milling abutments without FDA registration. Pair that freedom with Preat’s new ASC Ti blanks, and you gain advanced screw channel control that keeps even more cases in-house. More customization. More customer satisfaction. More growth.
Streamline Cases Efficiently
Thanks to Preat’s advanced scan acceptance, reverse scan body protocol and variable angulated screw channels, you can avoid painful and time-consuming steps in the digital case collaboration. Create better restorations faster all while enhancing your customer’s experience.
Seamless Workflow Integration
Preat’s PNP-compatible solutions work with popular CAD/CAM software, scanners and milling equipment — so you can scale without reinventing your workflow.
Partners & Integrations
CAM Production
CAD Design



Compatible with All Major Implant Systems